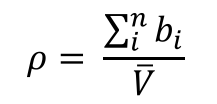| Version 3 (modified by ajj, 7 years ago) (diff) |
|---|
KEMM37 Lab 1B - SANS Data Analysis using SasView
| Table of Contents |
|---|
| 3. Fitting SANS data |
| 3.1 Background Information |
| 3.2 Calculating Scattering Length Density |
| 3.3 Loading and Plotting the data |
| 3.4 Fitting the data |
| Resources |
3. Fitting SANS data
This part of the exercise will use real SANS data taken from a study of surfactant self assembly in deep eutectic solvents (DES).
TASK 10: Restart SasView
Before starting this part of the exercise, you should have a clean SasView instance. Quit SasView and restart it.
3.1 Background Information
Deep eutectic solvents are a class of ionic liquids formed from a hydrogen bond donor and a halide salt. At a certain mixture ratio, the eutectic mixture, the melting point is significantly depressed to values below room temperature.
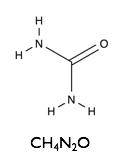
Here we will examine the self-assembly of a surfactant, sodium dodecyl sulfate (SDS) in the deep eutectic solvent formed from a 1:2 molar ratio mixture of choline chloride and urea.
| Sodium Dodecyl Sulfate | Choline Chloride | Urea |
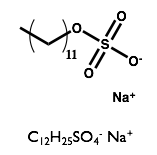 | 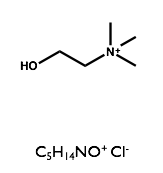 | 
|
TASK 11: Download the SANS data : kemm37SasViewTutorialData.zip and unzip the file in a known location on your filesystem. Note where you have placed the data.
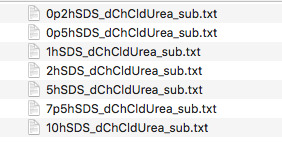
You should now have a folder containing a set of files named as follows:
The data are SANS curves collected on SANS2D at ISIS and D22 at ILL for samples of protonated (normal) SDS in 1:2 d9-choline chloride:d4-urea. This sample was chosen to give maximum contrast and minimum background signal from incoherent scattering. There were 7 samples with 0.2 wt%, 0.5 wt%, 1.0 wt%, 2.0 wt%, 5.0 wt%, 7.5 wt% and 10 wt% of SDS in the DES with the filenames corresponding to each sample given below:
| Surfactant Concentration (wt%) | Data File |
| 0.2 | 0p2hSDS_dChCldUrea_sub.txt |
| 0.5 | 0p5hSDS_dChCldUrea_sub.txt |
| 1.0 | 1hSDS_dChCldUrea_sub.txt |
| 2.0 | 2hSDS_dChCldUrea_sub.txt |
| 5.0 | 5hSDS_dChCldUrea_sub.txt |
| 7.5 | 7p5hSDS_dChCldUrea_sub.txt |
| 10.0 | 10hSDS_dChCldUrea_sub.txt |
All the data files have been processed to 1D scattering curves with the solvent background subtracted to leave only the coherent scattering signal on absolute scale.
3.2 Calculating Scattering Length Density
The scattering length density is given by
Scattering lengths of relevant elements:
| Element | Scattering Length (fm) |
|---|---|
| C | 6.646 |
| H | -3.739 |
| D | 6.671 |
| N | 9.36 |
| O | 5.803 |
| Cl | 9.577 |
Physical properties of the DES components:
| Component | Chemical Formula | Molecular Volume (Å3) | Density (g/cm3) |
|---|---|---|---|
| d9-Choline Chloride | C5H5D9NOCl | 210.77 | 1.17 |
| d4-Urea | CD4N2O | 75.55 | 1.41 |
TASK 12: Calculate the scattering length density (SLD) of a 1:2 mole ratio mixture of choline chloride and urea.
Use the information in the table above to calculate the SLD. There are multiple ways to do so, including:
- Calculating by hand
- Using a spreadsheet
- Using the SLD calculator built in to SasView (in the Tools menu).
- Using online calculators e.g. https://www.ncnr.nist.gov/resources/activation/
Try multiple ways and see if you get the same answer!
3.3 Loading and Plotting the data
You will now load the data into SasView and make a plot in order to visually inspect the scattering curves.
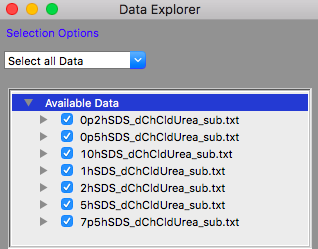
TASK 13: Click on the "Load Data" button in the Data Explorer
Locate the folder where you placed the data, select all the files in that folder and click "Open" in the dialog.
The Available Data section of the Data Explorer should look something like:
TASK 14: Plot the loaded data
Make sure that all the datasets have check marks next to them in the Available Data section of the Data Explorer, as shown above.
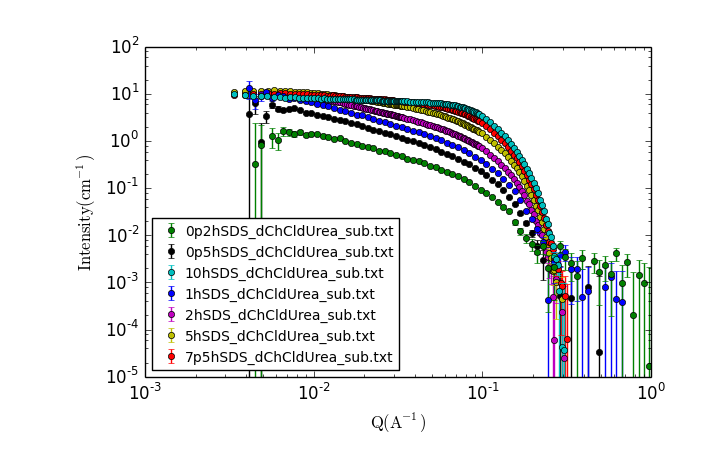
Click the "New Plot" button in the Data Explorer.
A new window should appear with a plot of the data that looks something like:
TASK 15: Examining the Data.
Visually inspect the data, zooming in and making additional plots as needed.
- What trends do you notice?
- What can you say about the possible solution structure from looking at the data?
3.4 Fitting the data
TASK 16: Fitting the lowest concentration data.
Select the lowest concentration data only in the data explorer by ensuring only 0p2hSDS_dChCldUrea_sub.txt has a check mark next to it and click “Send to" fitting.
- Select the model for the structure you predicted for this dataset.
- How does it compare to the data?
- Fill in parameters you know and adjust the others to see how close you get to the data.
- Select parameters to fit and run the fit by clicking "Fit" at the bottom of the fitting panel.
- Do you get a good fit?
- Are the parameters you get physically reasonable?
TASK 17: Fitting the other data, starting with the 7.5 wt% data set.
Repeat for other concentrations
- Does the same model fit all data?
- What is consistent between datasets? What is different?
Resources
- NIST SLD calculator https://www.ncnr.nist.gov/resources/activation/
- NIST Scattering Length and Scattering Cross Section Database https://www.ncnr.nist.gov/resources/n-lengths/
Attachments (10)
- kemm37_datafileslist.png (33.8 KB) - added by ajj 7 years ago.
- kemm37_sld.png (11.0 KB) - added by ajj 7 years ago.
- kemm37_dataplot.png (69.7 KB) - added by ajj 7 years ago.
- kemm37_loaddata.png (29.5 KB) - added by ajj 7 years ago.
- kemm37_des.png (16.7 KB) - added by ajj 7 years ago.
- kemm37_sds.png (12.5 KB) - added by ajj 7 years ago.
- kemm37_urea.png (10.9 KB) - added by ajj 7 years ago.
- kemm37_choline.png (10.8 KB) - added by ajj 7 years ago.
- kemm37_dataoperation.png (245.8 KB) - added by ajj 7 years ago.
- KEMM37SasViewTutorialData.zip (36.5 KB) - added by ajj 7 years ago.
Download all attachments as: .zip